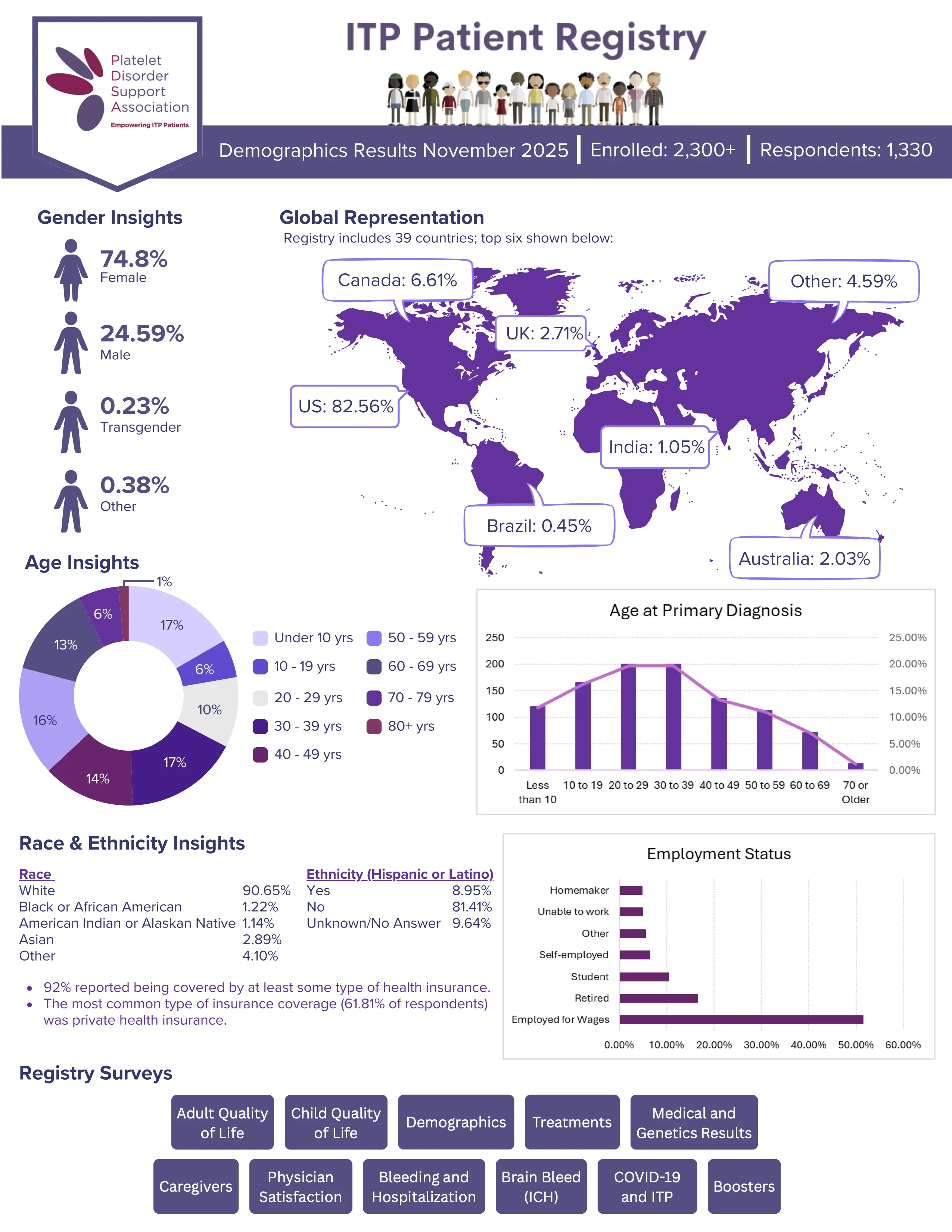
Surveys Available Within the Registry
Bleeding and Hospitalization
Survey
Medical and Genetics Results
Survey
Brain Bleed (ICH)
Survey
Adult Quality of Life
Surveys
Child Quality of Life
Surveys
Treatment
Survey
Caregiver
Survey
Demographic
Survey
COVID-19 and ITP
Survey
Booster
Survey
Physician Satisfaction
Survey
Clotting and ITP
Survey
(coming soon)
Register Today to Help Advance Research
PDSA is proud to have been chosen by the National Organization for Rare Disorders (NORD) to participate in its cooperative project with the U.S. Food And Drug Administration (FDA) known as the NORD Natural History Study Project.
By registering with PDSA’s global ITP National History Study Registry you’ll join thousands of patients living with ITP from around the world to advance research and improve the quality of life for ITP patients. At PDSA, we know you are the key to unlock a positive future for those with ITP.
What is the ITP Natural History Study Registry?
The ITP Natural History Study Registry is an international patient-consented registry of individuals with ITP and other platelet disorders. The registry aims to collect data on the natural progression of ITP and other platelet disorders, enabling PDSA to gather data on diagnosis and treatment, management of care, quality of life, and clinician reporting. The registry is administered by PDSA and overseen by NORD and a committee of leading hematologists, ITP patients and caregivers.
Why Register?
Registries provide healthcare professionals and researchers with first-hand information about people with certain conditions like ITP and other platelet disorders, individually and as a disease population, to increase their understanding of the condition over time.
Rare diseases like ITP have posed unique challenges to researchers and drug developers because of small patient populations, lack of data, clinical endpoints that are often unclear, and clinical trial enrollment and retention challenges. NORD’s Natural History Studies project empowers patients and families while helping eliminate some of the uncertainty in rare disease research, making way for progress.
Advance Research
Natural history studies are longitudinal studies that aim to fill research gaps, which help medical researchers better understand how diseases progress over time. They yield vital information essential to clinical trial designs, such as biomarkers, demographics, important clinical symptoms, genetic and environmental variables, and patient perspectives.
Make a Difference
Your participation in the ITP Natural History Study Registry is the first step to increase what we know about ITP and other platelet disorders, help healthcare professionals improve treatment, and allow researchers to design better studies on a particular condition, including development and testing of new treatments. The more patients register the more data researchers will have to further their work, which can accelerate research into new therapies for ITP and potentially a cure for the future.
Enroll now in PDSA’s National Patient Registry dedicated to those who have a platelet disorder such as ITP to advance research and improve the quality of life for patients and their caregivers.
For more information, contact PDSA at 440-746-9003 (877-528-3538 toll free) or email pdsa@pdsa.org.
Your identity will be protected in this secure, confidential HIPAA (Health Insurance Portability and Accountability Act) compliant registry, and there is no cost for you to participate.
Thank you for helping make the world of ITP a more manageable place to be!